A) The compound can have cis-trans isomers.
B) The compound will have substituents in its meta positions.
C) The compound will have substituents bonded to its 1,4 positions.
D) The compounds will have substituents in its meta positions and bonded to its 1,4 positions.
F) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
In which of the following hydrocarbons is the bond angle about the carbon atom approximately 120°?
A) alkanes
B) alkenes
C) alkynes
D) none of these
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How should a benzene ring with a methyl substituent and a hydroxyl substituent be named according to IUPAC nomenclature?
A) hydroxymethylbenzene
B) hydroxytoluene
C) methylphenol
D) methylhydroxybenzene
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which product is formed by the chlorination of 2-methyl-1-butene?
A) 1,1-dichloro-2-methylbutane
B) 1,2-dichloro-2-methylbutane
C) 2-chloro-2-methylbutane
D) 1-chloro-2-methylbutane
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the charge on the organic intermediate formed during hydrohalogenation?
A) −2
B) −1
C) +1
D) +2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four patterns can be noted for certain organic reactions: Pattern 1: Add a proton.
Pattern 2: Take a proton away.
Pattern 3: Reaction between an electrophile and nucleophile to form a new covalent bond.
Pattern 4: Reaction of a proton donor with a carbon-carbon double bond to form a new covalent bond.
To which pattern does the following reaction belong? 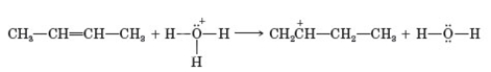
A) Pattern 1
B) Pattern 2
C) Pattern 3
D) Pattern 4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the substance represented by the following model. 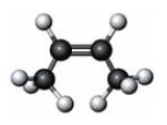 The physical properties of this substance would most closely represent the corresponding member of which functional group class?
The physical properties of this substance would most closely represent the corresponding member of which functional group class?
A) an alkyne
B) an alcohol
C) an aldehyde
D) a carboxylic acid
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following characteristics is associated with the presence of cis-trans isomerism?
A) the presence of double bonds
B) the presence of ring systems
C) the presence of both double bonds and ring systems
D) the absence of both double bonds and ring systems
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Including cis-trans isomers, how many stereoisomers are possible for an alkene that has the molecular formula C4H8?
A) 2
B) 6
C) 4
D) 5
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of intermediate is generated during the acid-catalyzed hydration of an alkene?
A) an oxonium ion
B) a carbocation
C) both an oxonium ion and a carbocation
D) neither an oxonium ion nor a carbocation
F) B) and D)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following observations was troublesome to 19th-century chemists in the determination of benzene's structure?
A) Benzene contains more hydrogen atoms than expected.
B) Benzene does not undergo combustion.
C) Benzene does not react similarly to alkenes.
D) Benzene reacts similarly to alkenes.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct IUPAC name of the following compound? 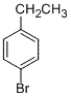
A) 1-ethyl-4-bromobenzene
B) 4-ethyl-1-bromobenzene
C) 1-bromo-4-ethylbenzene
D) 4-bromo-1-ethylbenzene
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A student named a particular compound 2-propyl-1-butene. Assuming that the student's choice actually corresponded to the correct distribution of the double bond and the substituents, what is the correct IUPAC name for this compound?
A) 2-propyl-1-butene
B) 2-ethyl-1-pentene
C) 3-methyl-2-pentene
D) none of these
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions requires a transition metal catalyst?
A) halogenation
B) hydrohalogenation
C) hydrogenation
D) hydration
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions involves the addition of different groups to both the ends of a double bond?
A) bromination
B) hydrogenation
C) both bromination and hydrogenation
D) neither bromination nor hydrogenation
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Examine the following reaction. 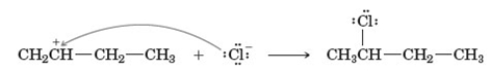 This reaction occurs in which step of the hydrohalogenation reaction mechanism?
This reaction occurs in which step of the hydrohalogenation reaction mechanism?
A) 1
B) 2
C) 3
D)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the monomeric unit of PVC.
A) propylene
B) acrylonitrile
C) chloroethene
D) 1,1-dichloroethylene
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What happens when the following substance reacts with water in the presence of concentrated sulfuric acid? 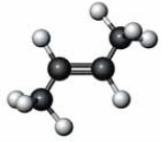
A) An alkyne is produced.
B) An alcohol is produced.
C) The substance is converted to its cis isomer.
D) The substance is converted to its trans isomer.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is an ether?
A) aniline
B) anisole
C) phenol
D) toluene
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following catalysts are used for producing low-density polyethylene from ethylene?
A) peroxide initiators
B) Ziegler-Natta catalysis
C) either peroxide initiators or Ziegler-Natta catalysis
D) neither peroxide initiators nor Ziegler-Natta catalysis
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 184
Related Exams