A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
The name of the 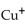 ion is
ion is
A) copper(I) .
B) cobalt.
C) copper(II) .
D) copper.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the electronegativity difference between elements X and Y is 2.1, the bond between the elements X-Y is
A) nonpolar ionic.
B) impossible.
C) ionic.
D) polar covalent.
E) nonpolar covalent.
G) B) and E)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The strongest interactions between molecules of ammonia, 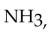 are
are
A) polar covalent.
B) ionic bonds.
C) dispersion forces.
D) dipole-dipole attractions.
E) hydrogen bonds.
G) A) and C)
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
What is the formula of a compound that contains 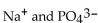 ions?
ions?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The types of compounds that use prefixes in their names are
A) ionic compounds.
B) compounds that contain polyatomic ions.
C) polyatomic ions.
D) covalent compounds.
E) ionic compounds involving transition metals.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The shape of the ammonia molecule  is
is
A) linear.
B) hexagonal.
C) square.
D) octagonal.
E) trigonal pyramidal.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for aluminum nitrite?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds contains an ionic bond?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The water molecule has a dipole with the negative portion
A) pointing from the oxygen through the hydrogen atoms.
B) localized between the hydrogen atoms.
C) localized on one of the hydrogens.
D) pointing toward the oxygen atom.
E) surrounding the molecule.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ionic charge of an ion with 13 protons and 10 electrons?
A) 3-
B) 2+
C) 2-
D) 3+
E) 1+
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula of copper(I) sulfide is
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) Cu2(So4) 3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In ionic compounds, ________ lose their valence electrons to form positively charged ________.
A) nonmetals; anions
B) metals; anions
C) metals; polyatomic ions
D) nonmetals; cations
E) metals; cations
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The strongest interactions between molecules of hydrogen, 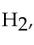 are
are
A) dispersion forces.
B) hydrogen bonds.
C) ionic bonds.
D) dipole-dipole attractions.
E) polar covalent.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
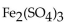 is called
is called
A) iron(III) sulfate.
B) iron(II) sulfate.
C) iron trisulfate.
D) iron sulfate.
E) diiron trisulfate.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An anion always
A) has a negative charge.
B) contains a metal and a nonmetal.
C) has a positive charge.
D) forms covalent bonds.
E) contains a group of two or more atoms with a positive charge.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements does NOT exist as a diatomic molecule?
A) hydrogen
B) nitrogen
C) carbon
D) oxygen
E) chlorine
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following polyatomic ions has a 3- ionic charge?
A) phosphate
B) hydroxide
C) sulfate
D) nitrate
E) bicarbonate
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of 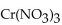 ?
?
A) chromium(III) nitrite
B) chromium trinitrate
C) chromium nitrate
D) chromium(III) nitrate
E) chromium trinitrite
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Valence electrons are electrons located
A) in the nucleus of an atom.
B) in the outermost energy level of an atom.
C) in the first three shells of an atom.
D) in the innermost energy level of an atom.
E) throughout the atom.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 83
Related Exams