A) I
B) II
C) III
D) More than one of these choices.
E) None of these choices.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound does NOT possess a plane of symmetry? 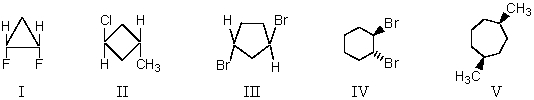
A) I,II and V
B) I,III and IV
C) II,III and IV
D) III and IV
E) V
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the percent composition of a mixture of (S) -(+) -2-butanol,[ ] ![What is the percent composition of a mixture of (S) -(+) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup> = +13.52,and (R) -(-) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup>= -13.52,with a specific rotation [ \alpha ] <sub> </sub> <sup> </sup> = +6.76? A) 75% (R) ,25% (S) B) 25% (R) ,75% (S) C) 50% (R) ,50% (S) D) 67% (R) ,33% (S) E) 33% (R) ,67% (S)](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d9b_d137_9180_43bb3b42d530_TB5902_00.jpg) = +13.52,and (R) -(-) -2-butanol,[ ]
= +13.52,and (R) -(-) -2-butanol,[ ] ![What is the percent composition of a mixture of (S) -(+) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup> = +13.52,and (R) -(-) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup>= -13.52,with a specific rotation [ \alpha ] <sub> </sub> <sup> </sup> = +6.76? A) 75% (R) ,25% (S) B) 25% (R) ,75% (S) C) 50% (R) ,50% (S) D) 67% (R) ,33% (S) E) 33% (R) ,67% (S)](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d9b_d138_9180_f57a8c5a1207_TB5902_00.jpg) = -13.52,with a specific rotation [ ]
= -13.52,with a specific rotation [ ] ![What is the percent composition of a mixture of (S) -(+) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup> = +13.52,and (R) -(-) -2-butanol,[ \alpha ] <sub> </sub> <sup> </sup>= -13.52,with a specific rotation [ \alpha ] <sub> </sub> <sup> </sup> = +6.76? A) 75% (R) ,25% (S) B) 25% (R) ,75% (S) C) 50% (R) ,50% (S) D) 67% (R) ,33% (S) E) 33% (R) ,67% (S)](https://d2lvgg3v3hfg70.cloudfront.net/TB5902/11eaa4bc_3d9b_d139_9180_0798c89ef343_TB5902_00.jpg) = +6.76?
= +6.76?
A) 75% (R) ,25% (S)
B) 25% (R) ,75% (S)
C) 50% (R) ,50% (S)
D) 67% (R) ,33% (S)
E) 33% (R) ,67% (S)
G) All of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw a dash-wedge structure for (R)-3-methyl-5-hexen-3-ol
Correct Answer

verified
Correct Answer
verified
True/False
A sample consisting of the pure R enantiomer of a compound will always rotate plane-polarized light in a clockwise direction.
B) False
Correct Answer

verified
Correct Answer
verified
Essay
Draw a dash-wedge structure for (2R,3S)-2-bromo-3-chlorohexane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule is a meso compound? 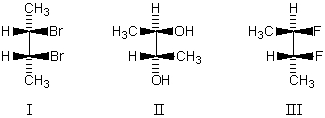
A) I
B) II
C) III
D) More than one of these choices.
E) None of these choices.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a dash-wedge structure for (1S,2S,5R)-5-methyl-2-propylcyclohexanol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct stereochemistry for the indicated stereocenters in (+) -camphor? 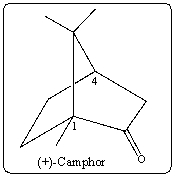
A) 1R,4R
B) 1R,4S
C) 1S,4S
D) 1S,4R
E) None,molecule is not chiral.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecules below are: 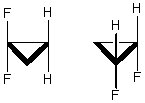
A) constitutional isomers.
B) enantiomers.
C) diastereomers.
D) identical.
E) stereoisomers.
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
A diastereomeric mixture can be very difficult to purify as both isomers have the same physical properties.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
I and II are: 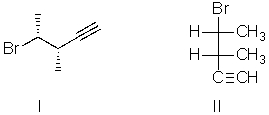
A) constitutional isomers.
B) enantiomers.
C) identical.
D) diastereomers.
E) not isomeric.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
I and II are: 
A) constitutional isomers.
B) enantiomers.
C) identical.
D) diastereomers.
E) not isomeric.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
A chiral molecule was found to have a specific rotation of +19.5,thus we can conclude that the enantiomer of this compound will have a rotation that is equal and opposite in value.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecules below are: 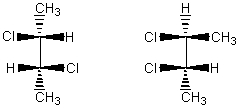
A) enantiomers.
B) diastereomers.
C) constitutional isomers.
D) two different conformations of the same molecule.
E) not isomeric.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a solution of a compound (30.0 g/100 mL of solution) has a measured rotation of +15º in a 2.0 dm tube,the specific rotation is:
A) +50
B) +25
C) +15
D) +7.5
E) +4.0
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
I and II are: 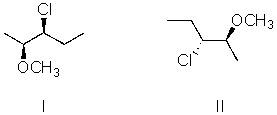
A) constitutional isomers.
B) enantiomers.
C) identical.
D) diastereomers.
E) not isomeric.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a dash-wedge structure for (3S,5R)-5-chloro-3-isopropylcyclohex-1-ene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many stereogenic centers are in the following compound: 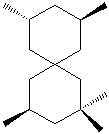
A) 1
B) 3
C) 4
D) 5
E) none of these choices
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound would show optical activity?
A) cis-1,4-Dimethylcyclohexane
B) trans-1,4-Dimethylcyclohexane
C) cis-1,4-Dimethylcycloheptane
D) trans-1,4-Dimethylcycloheptane
E) More than one of these choices.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 179
Related Exams